Mobile therapy
THAT WORKS
Breathe easier knowing they will, too
The Monarch airway clearance system from Hillrom uses revolutionary technology to take High Frequency Chest Wall Oscillation (HFCWO) therapy mobile. It uses targeted kinetic energy and airflow to help thin and mobilize secretions—all while your patients move freely throughout their busy days.
And with the Connex app and Health Portal, you can view your patients’ therapy session data any time—helping you create more detailed care plans, and helping them manage their daily care routines.
The Monarch system combines freedom and connection to help your patients take control of their therapy—and their lives.
How it works
The Monarch system’s eight Pulmonary Oscillating Discs (PODs) are positioned to deliver targeted kinetic energy to the upper and lower lungs. This targeted therapy generates airflow, helping to thin mucus and mobilize secretions from the small to large airways, where they can be coughed or suctioned out—helping improve your patients’ airway health.1-4
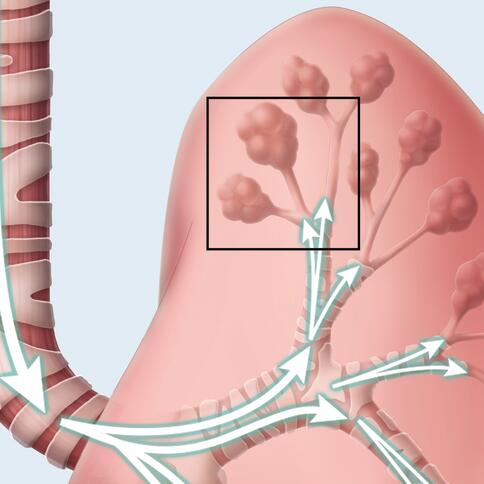
The PODs operate in a pattern designed to loosen mucus and help clear your patients’ airways.
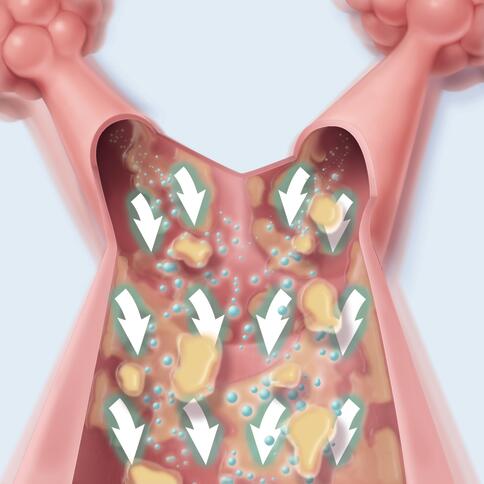
Airflow moves the mucus from small to large airways, where they can be coughed or suctioned out.
Clinical results
Data from a clinical evaluation of the Monarch airway clearance system by Leemans G., et al., were published in Pediatric Pulmonology in 2020. The researchers reported the Monarch system was comparable to The Vest airway clearance system for sputum production.
Post-therapy evaluations of the Monarch system showed statistically significant improvement in Brody scores and FRI results, suggesting the device’s relative airway clearance effectiveness.5
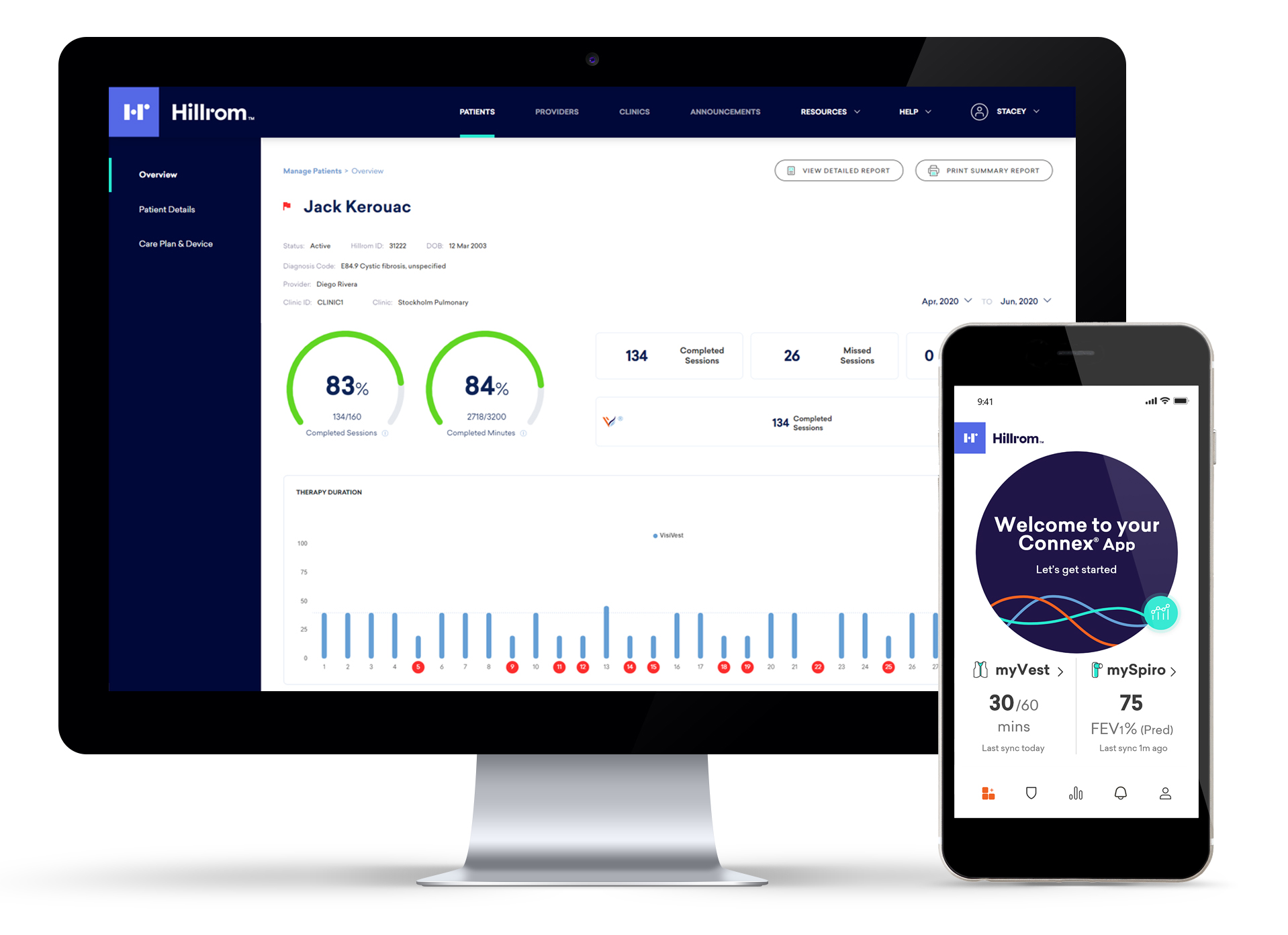
Connection you can feel good about
When you are closely connected to your patients, you can make better decisions together. That’s why the Monarch system integrates with the Connex app and Health Portal—giving you both easy access to daily therapy session results. Your patients can also use the app to track everything from medications and Pulmonary Function Tests (PFTs) to nutrition, exercise and more—rewarding healthy behaviors and empowering them to feel their best.
Indications and important safety information
Indications for Use6
The Monarch airway clearance system is intended to provide Airway Clearance Therapy and promote bronchial drainage where external manipulation of the thorax is the physician’s choice of treatment. It is indicated for patients having difficulty with secretion clearance, or the presence of atelectasis caused by mucus plugging. The Monarch airway clearance system is intended to be used in the Home Care environment by patients, 15 years and older.
Contraindications6
If any patient conditions exist that could cause the use of the Monarch airway clearance system, to present a risk to the patient, do not use the unit except as directed by a physician. Death or serious injury could occur.
The Monarch airway clearance system, is contraindicated if these conditions are present:
- The Monarch device uses Pulmonary Oscillating Discs (PODs) that create a magnetic field which is present whether the device is turned on or off. Due to the presence of the magnetic field, people who have an active implantable medical device, such as any of the following, are contraindicated (if they cannot keep the susceptible component at least 6 inches (15.5 cm) away from the Monarch device:
- Pacemakers
- Neurostimulators
- Infusion Pumps
- Circulatory Support Devices
- Implantable Cardioverter Defibrillators (ICDs)
- Cochlear Implants
- Head and/or neck injury that has not yet been stabilized.
- Active hemorrhage with hemodynamic instability.
Warnings6
The Monarch airway clearance system warnings include:
- Patients that may have difficulty clearing secretions from the upper airway (such as those with DMD or other advanced neuromuscular or neurological disorders) may require specialized therapy regimens involving manually or mechanically assisted coughing or other techniques in conjunction with Monarch airway clearance system. Please consult your physician to determine if additional therapy is appropriate.
- The Monarch airway clearance system has been prescribed by your physician for your use only. Do not let others try on your device, whether it is turned on or off. It should never be worn or used by someone with an active implantable medical device, due to the presence of the magnetic field created by the Monarch Pulmonary Oscillating Discs (PODs). See the Contraindications section in the manual for a further description of an active implantable medical device.
For a complete list of Contraindications and Warnings, please refer to the User Manual, or call us at 1-800-426-4224.
References
- King M, Phillips D, Gross D, Vartian V, Chang HK, Zidulka A. Enhanced tracheal mucus clearance with high frequency chest wall compression. Am Rev Respir Dis, 1983; 128:511-5.
- Dosman CF and Jones RL. High-frequency chest compression: a summary of the literature. Can Respir J, 2005. 12(1): p. 37-41.
- Freitag L, et al. Removal of excessive bronchial secretions by asymmetric high-frequency oscillations. J Appl Physiol 1989; 67: 614-9.
- McCarren B, Alison JA. Physiological effects of vibration in subjects with cystic fibrosis. Eur Resp J 2006; 27: 1204-9.
- Leemans G, Belmans D, Van Holsbeke C, et al. The effectiveness of a mobile high‐frequency chest wall oscillation (HFCWO) device for airway clearance. Pediatric Pulmonology. 2020;55: 1984–1992. https://doi.org/10.1002/ppul.24784
- The Monarch Airway Clearance System Model 1000 User Manual (195292).
Monarch and The Vest are trademarks of Baxter International, Inc. or its subsidiaries.
202762 rev 6 21-OCT-2022 ENG – US
