MADE TO MOVE
like you
Freedom to move about your life
It can be hard to fit therapy into your life, even though you know how important it is. By allowing you to move freely, the Monarch airway clearance system opens a new world of possibilities for doing your therapy, your way.
We’re excited about that new world—because for nearly 30 years, helping people with chronic respiratory diseases and conditions has been a big part of who we are at Hillrom.

Meet the mind behind the Monarch system
Marten De Vlieger and his sister were both born with cystic fibrosis (CF). As they grew up, their mother had to manually perform chest physiotherapy (CPT) to them, to help clear their airways.
But Marten envisioned a life with more independence. He created a mobile therapeutic device to provide him with the airway clearance therapy he needed. It freed him to travel, to pursue his love of kitesurfing, to have adventures, and to raise his own family.
And when Marten told us that he wanted others to experience the flexibility and control he’d claimed for himself, we gladly partnered with him to develop the Monarch airway clearance system.
Learn more about Marten by following him on Instagram – @cf_adventurerlife
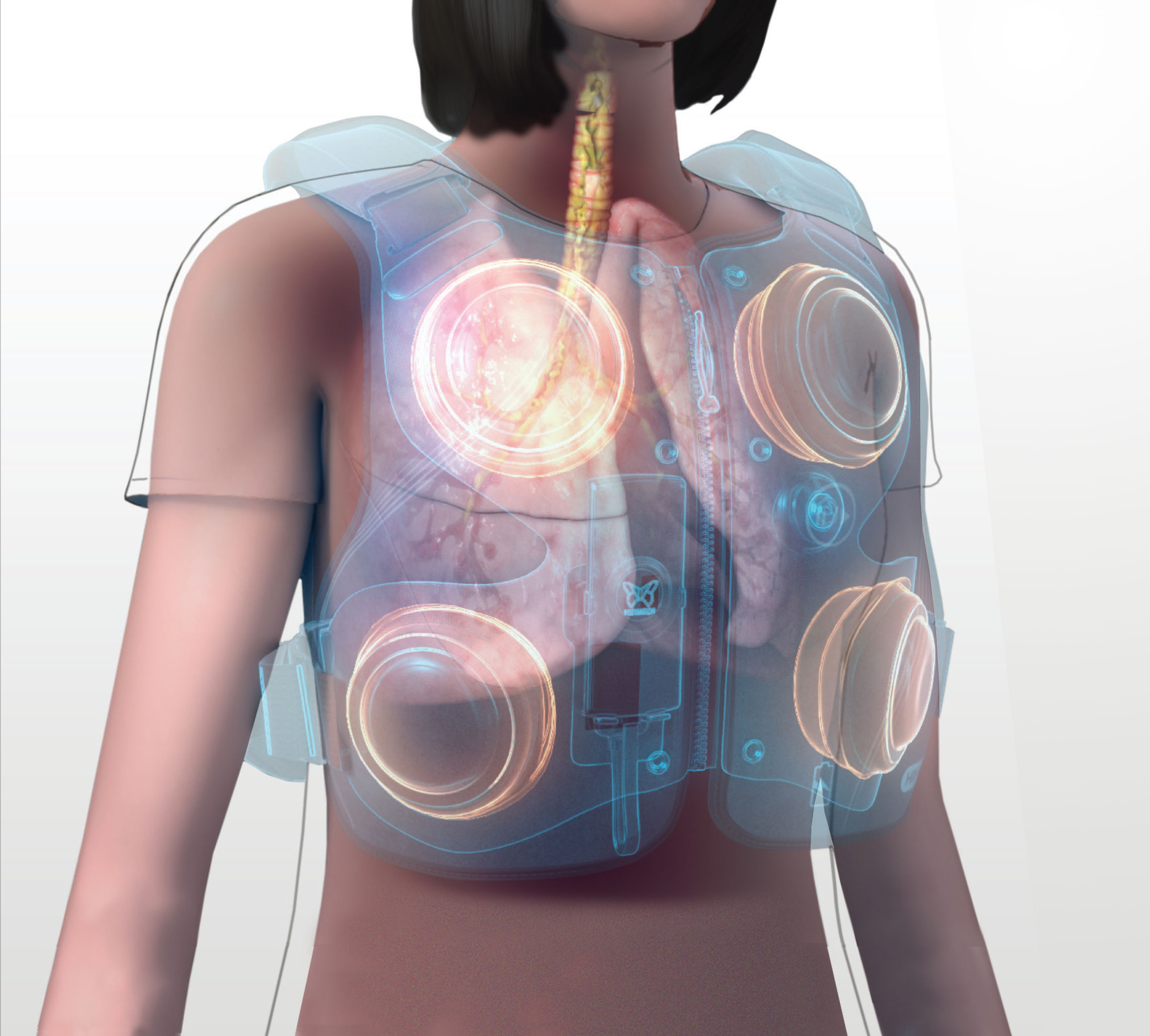
Mobility powered by POD technology
The Monarch system uses Pulmonary Oscillating Discs (PODs) to generate oscillations and targeted kinetic energy to your lungs. This airway clearance therapy helps thin mucus and increase airflow, mobilizing secretions from the airways while you’re going about your day. So you no longer have to put your life on hold for therapy.
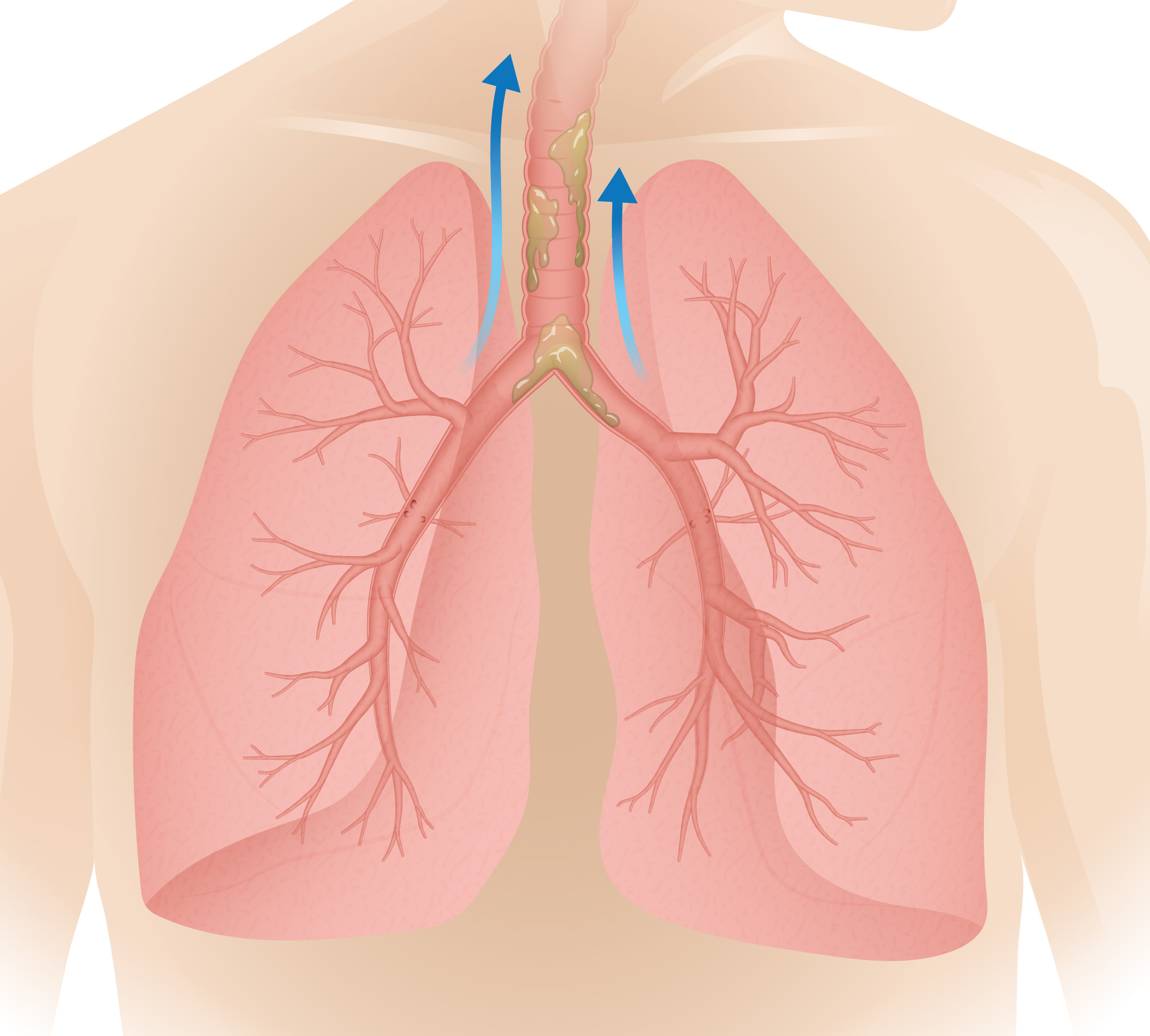
Front and back PODs operate in a pattern to optimize oscillatory effect to the airways.
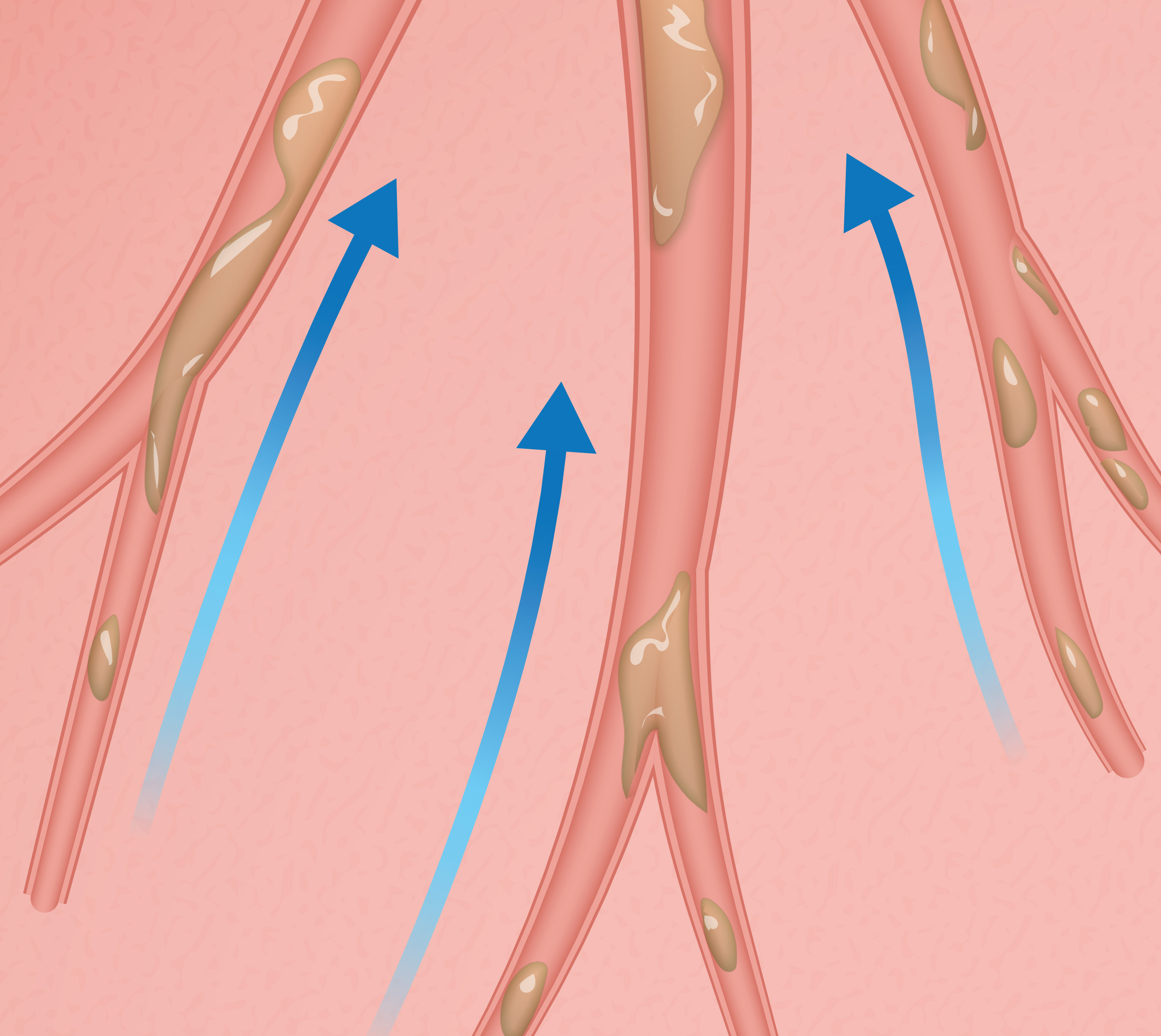
Oscillating PODs deliver targeted kinetic energy and generate airflow in the lungs to loosen mucus.
Air gets behind the mucus to move it to the large airways.
Your one-stop destination for feeling your best
You want to live your healthiest life every day. And you know the key to that is doing everything your doctor prescribes. But staying on top of it can be complicated. That’s why we made the Connex app. It simplifies managing your health, with one place to go to get motivated and keep track of everything, from your airway clearance therapy sessions to medications, nutrition, exercise and more.
Designed with you in mind
Sportswear-inspired design is made for freedom on mobility. Its quiet operation enables easy conversation.¹ Fun colors and patterns let patients personalize their device.
Graphite Orange
Zesty Pink
Techno Red
Ocean Blue
Adventure Green
What’s included

Mobile garment
with 8 independently operating PODs delivering targeted kinetic energy.

Pendant control
is intuitive and simple to use.

Vest shell
in your choice of five colors; washable & dryable.

Rolling carrying case
with retractable handle and backpack straps looks like ordinary luggage, and makes it easy to travel with the device.

Rechargeable battery
powers therapy on the move.

Power adapter and cord
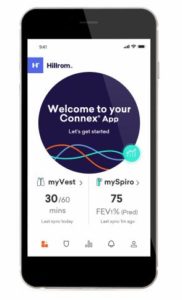
Connex connectivity
one-stop control center where you can manage your daily care routine.
Download the Connex mobile app on your smart phone by searching for “Connex” in the Google Play or App Store.
Indications and important safety information
Indications for Use²
The Monarch airway clearance system is intended to provide Airway Clearance Therapy and promote bronchial drainage where external manipulation of the thorax is the physician’s choice of treatment. It is indicated for patients having difficulty with secretion clearance, or the presence of atelectasis caused by mucus plugging. The Monarch airway clearance system is intended to be used in the Home Care environment by patients, 15 years and older.
Contraindications²
If any patient conditions exist that could cause the use of the Monarch airway clearance system, to present a risk to the patient, do not use the unit except as directed by a physician. Death or serious injury could occur.
The Monarch airway clearance system, is contraindicated if these conditions are present:
- The Monarch device uses Pulmonary Oscillating Discs (PODs) that create a magnetic field which is present whether the device is turned on or off. Due to the presence of the magnetic field, people who have an active implantable medical device, such as any of the following, are contraindicated (if they cannot keep the susceptible component at least 6 inches (15.5 cm) away from the Monarch device:
- Pacemakers
- Neurostimulators
- Infusion Pumps
- Circulatory Support Devices
- Implantable Cardioverter Defibrillators (ICDs)
- Cochlear Implants
- Head and/or neck injury that has not yet been stabilized.
- Active hemorrhage with hemodynamic instability.
Warnings²
The Monarch airway clearance system warnings include:
- Patients that may have difficulty clearing secretions from the upper airway (such as those with DMD or other advanced neuromuscular or neurological disorders) may require specialized therapy regimens involving manually or mechanically assisted coughing or other techniques in conjunction with Monarch airway clearance system. Please consult your physician to determine if additional therapy is appropriate.
- The Monarch airway clearance system has been prescribed by your physician for your use only. Do not let others try on your device, whether it is turned on or off. It should never be worn or used by someone with an active implantable medical device, due to the presence of the magnetic field created by the Monarch Pulmonary Oscillating Discs (PODs). See the Contraindications section in the manual for a further description of an active implantable medical device.
For a complete list of Contraindications and Warnings, please refer to the User Manual, or call us at 1-800-426-4224.
- Sound testing per International Standard IEC 60601-1, 3rd Edition at a distance of 30 cm. Sound testing results found the Monarch System operates at a level at or below that considered as a “normal conversation”; reference https://www.nidcd.nih.gov/health/noise-induced-hearing-loss.
- The Monarch Airway Clearance System Model 1000 User Manual (195292).
Hillrom, Monarch, and Connex are trademarks of Baxter International Inc., or its subsidiaries.
202759 rev 7 14-OCT-2022 ENG – US
